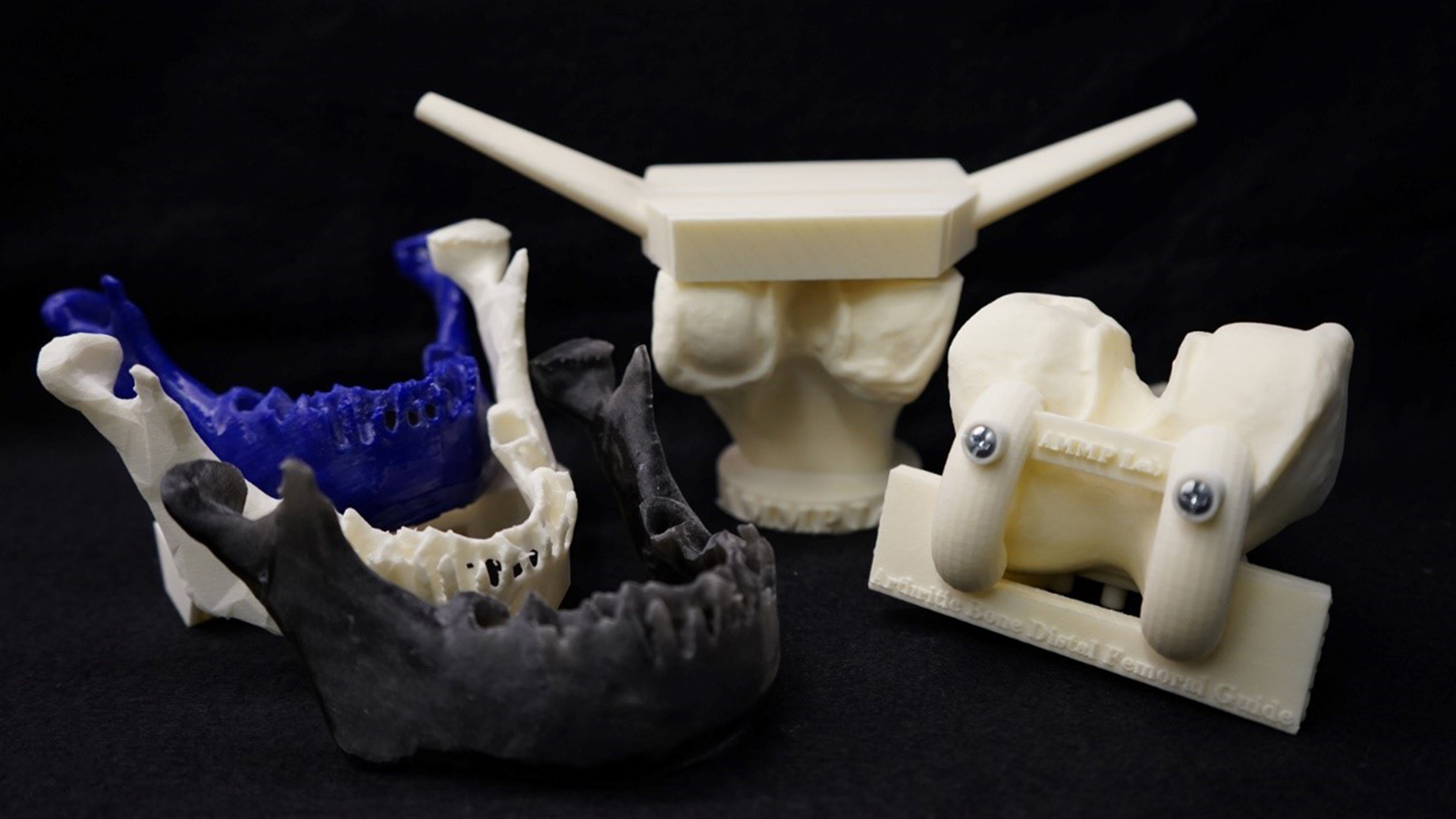
The U.S. Food and Drug Administration (FDA) posted a discussion paper on 3D Printing Medical Devices at the Point of Care (PoC).
The discussion paper provides background information on 3D printing and proposes potential PoC manufacturing scenarios for public comment. The discussion paper does not constitute guidance; instead, its purpose is to gather feedback from the public to inform future policy development.
The discussion paper:
- Considers relevant background, including terminology, the FDA regulation of devices and 3D printing, and how capabilities at a 3D printing facility factor into device safety and effectiveness
- Identifies challenges presented by 3D printed medical devices at PoC and presents a potential approach for regulatory oversight under various scenarios to inform future policy development
- Poses questions to facilitate public comment
[hubspot type=cta portal=5268583 id=146198cc-1d0a-4119-9ef5-4aa31e4ac862]
Public Comments Period
The FDA is seeking input on each of these topics and on 16 questions posed in the discussion paper. This discussion paper will be open for public comment for 60 days at https://www.regulations.gov under Docket Number FDA-2021-N-1272.
Questions?
If you have questions about 3D printing of medical devices at point of care, contact the Division of Industry and Consumer Education.


