The ability to proactively use 3D digital data in the medical space really came to fruition with the start of additive manufacturing in the late 1980s. Since then, stunning advances have occurred, especially in mass personalization. Take, for example, the commercialization of Invisalign dental aligners in 1999 using indirect 3D printing of forms, and the creation of patient-matched in-ear hearing aid cases by Siemens in the early 2000s, with millions of patients treated. From the very first 3D printed anatomical model in 1988 at UCLA, the evolution of patient-specific 3D models and surgical guide software by Materialise in the 1990s, and then Medical Modeling providing bespoke services in the early 2000s, 3D data has been successfully used by thousands of surgeons.
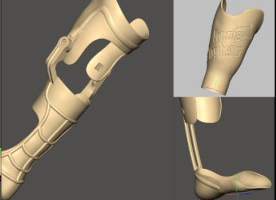
Use of 3D scan data of has also enabled the evolution of patient-specific 3D printed prosthetics over the last 30 years, and more recently, new inventions such as ‘dentures in a day’.
As 3D printing technology, materials, and software have improved so we see medical 3D printing applications leap forward. As metal 3D printing and materials have advanced so have solutions for healthcare. For example, the first-ever reported surgical implantation of a patient-specific 3D printed titanium jaw implant occurred in 2012. 3D printed spinal implants, and 3D printed knee and hip joints have also advanced to commercial availability across the past decade.
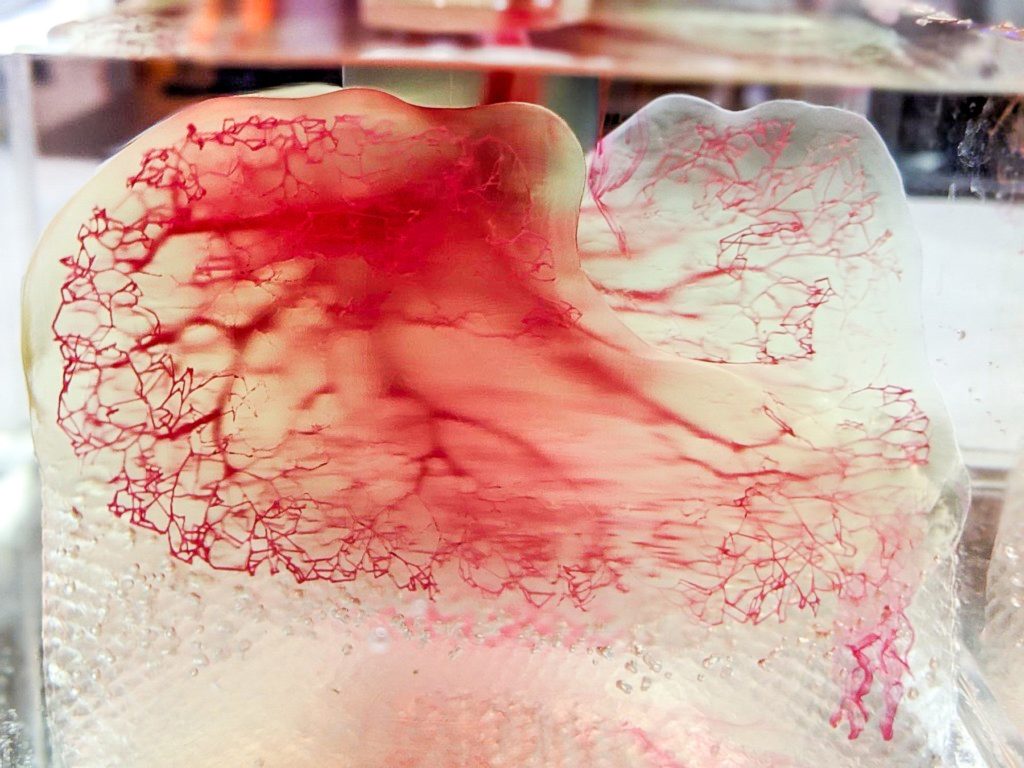
Recently tissue or 3D bioprinting has become a hot research topic. In fact in June 2022, United Therapeutics, in partnership with 3D Systems, revealed a fully 3D printed lung scaffold at the LIFE ITSELF Conference. During the session Dr. Martine Rothblatt, chairperson and CEO of United Therapeutics, stated “…we hope to have these personalized, manufactured lungs cleared for human trials in under five years.”
The use of 3D data in medicine seems to have unlimited potential. There’s a breathless “Even the sky is not the limit” feel to it. But in reality nothing is further from the truth. New innovations in 3D medical devices can take years, even decades, to become accepted and cleared. There are barriers, and challenges, to be overcome, and none of them are simple.
The Knowledge Gap
Despite the best effort of marketers in the past to say otherwise, 3D printing is never just about ‘Press the Print Button’. Software has been instrumental in advancing 3D digitization in just about every industry, but even then knowledge and skills in the specific application are still required.
Patient-matched prosthetics have been evolving since 3D printing began, but even with a well-established workflow, high-functioning software, reliable 3D printing hardware, this particular application remains difficult to learn and has had slow uptake.
Cambre Kelly, PhD, VP of Research and Technology at restor3D, in her presentation at ASME’s AM Medical Summit in October 2021 said “[At first] we had print failure after print failure. Trying to take a desktop system and scale it up for production. Trying to figure out how to do process qualification around these. How do you do IQ, OQ, PQ on systems like this? How do you optimize the print area that you have to produce the most number of parts with the most uptime? Then how do you scale that up?”

Gaining that knowledge takes time and effort. It is an uphill grind of trial and error, of stitching together old and new workflows, software, materials and printing processes to develop new ones.
The 3D Data – Input
Capturing 3D data for medicine usually uses two key solutions – 3D surface scanning and medical image processing.
In 1992, Materialise developed Materialise Mimics software to handle the processing of medical image data of patients, and has remained very much on the forefront of that discipline with growing competition in the last decade from competitors such as Oqton’s D2P software.
3D surface scanning reaches back to the late 1990s. Coupled with a growing portfolio of OEM 3D scanners, these software products blazed the way in high-accuracy 3D reverse engineering and inspection solutions, mostly for engineering applications, but notably also for dental 3D scanning. However, in the most part these solutions had a high cost of access for customers and a steep learning curve.
In 2013, the Structure sensor by Occipital changed the 3D scanning landscape by providing a very cheap handheld 3D scanner that would work on an iPad and that started a revolution in correspondingly cheap 3D scanner software. However, with these solutions accuracy and resolution were reduced to 1-2 mm – good enough for some applications, but by no means good enough for high-precision medical devices.
“The Structure sensor started the roller-coaster ride in 3D scanning for prosthetics,” said Brent Wright, a certified Prosthetist and Orthotist by the American Board for Certification in Orthotics, Prosthetics and Pedorthics, practicing at Eastpoint Prosthetics. “We no longer needed high-priced 3D scanners and so accessibility and affordability improved for practitioners and patients.”

But for high accuracy, patient-specific 3D data, the use of medical image data was increasing and with it, complications, mostly connected to an ongoing knowledge gap by operators, and a lack of clear standards.
Dima Elissa, CEO and founder of VisMed3D, said during an ASME Panel on Digitalization in AM in October 2021, “I can’t tell you how many times I’ve received MRI DICOM data and the variability of quality and the lack of usability of it because people don’t understand what it needs to be in and what form and what resolution or context.”
The rise of many inexpensive 3D scanning software solutions as well as photogrammetry has made 3D data capture very accessible. For high-grade accuracy, prices rise as do learning curves. But once mostly solved, this then gave rise, even early on, to 3D data integration and interoperability issues.
The 3D Data Integration Gap
Materialise, and later Geomagic and RapidForm, quickly saw a need for software that would tackle ‘tricky’ 3D data. With AM came the standardization of the STL file format for 3D printing. And not every STL file was (or still is) created equally. Materialise Magics led the way in establishing usable workflows from 3D CAD data through to 3D printing. In recent years, inexpensive or even free STL editors have appeared, although I hear anecdotally that in this case you mostly pay for what you get.
In the most part, basic 3D data capture and integration is solved, although sometimes still expensive and still evolving. But even then, further software and data process integration still needs to occur, namely in FEA/simulation, Artificial Intelligence, QA software and processes, Big Data, and more.
As new lattice structures and topologies are being used in medical devices, Finite Element Analysis (FEA)-type simulation and computational modeling is becoming a necessity for the parts themselves. As more wearable devices are developed, such things as analysis of emmisions from such devices is becoming a requirement. To progress, FEA software will need to move beyond its roots of traditional of product engineering modeling and enable medical device researchers to conduct advanced testing through virtual anatomical models.
As expressed by ANSYS in its 2021 white paper on ‘Leveraging Engineering Simulation to Fast-Track Personalized Healthcare’.
“Engineering simulation provides a cost-effective, rapid and straightforward solution for modeling patients’ bodies and designing devices that interact optimally with the body. This allows healthcare providers to devise truly personalized treatment plans, as well as to predict health problems before they occur, enabling early intervention.”
The implementation of Artificial Intelligence (AI) is already making inroads to improve medical treatment, diagnosis and health trends. However, the requirement for big data – lots and lots of medical records – brings up ethical issues with privacy of patient data alongside the risks of biased or inaccurate data being used. On the 3D side, implementations of AI have taken longer to develop but are now being seen in applications such as Medical IP’s imaging and segmentation software.
QA technology and solutions have also been lagging in the 3D integration gap. In many cases, inspection results are written on a piece of paper, typed into a spreadsheet, imported from inspection devices or any of the myriad other inspection tools used for measuring the produced part. This results in quality data being stored in multiple places making it difficult to accurately use the correct data for the correct report, audit and compliance.
“Manufacturing Quality assurance software is now at the point where all start from the 3D model or 2D print that can be fully integrated and automated as a closed loop within the entire product development and production workflow,” said Sam Golan, CEO, High QA, a developer of integrated Quality Management Software (QMS) solutions. “The biggest issue is not about measuring the part correctly but how the measurements are recorded, where the inspected data resides, and what real value that data provides beyond ‘good or bad.’ For precision, patient-specific healthcare devices, this kind of integration is not just desirable but a necessity.”
The other gaps – standards, regulations and infrastructure.
Alongside technology gaps, there are other barriers that need to be addressed in the digitalization of 3D data for healthcare, namely standards, regulations, new supply chain infrastructures and more.
“3D bioprinting offers tremendous promise in delivering patient-specific solutions. However, I believe there are still several gaps which the industry is working to address,” commented Katie Weimer, Vice President, Regenerative Medicine, 3D Systems. “Aside from the major technical challenge of bioprinting cellularized vascular tissues at human scale, we are also challenged by infrastructure. This includes gaps in: the regulatory pathway to take these products to market, the scientific standards these products need to meet, and also in the entire supply chain to support large volume 3D printing production of tissue and organ products at scale.”
As radical new solutions such as 3D bioprinting are developed so the infrastructure around them has to evolve. And sometimes it is not fast enough.
3D digitization in healthcare has always had great promise in medicine: It continually offers new and significant technological trajectories. Many gaps and shortcomings have been addressed. But there is far more yet to be achieved and in many areas a paradigm shift in technologies, regulations and data availability will be required.
To become part of this conversation and more, register now for the ASME AM Medical Industry Summit, Nov 1-3 2022 in Minneapolis, MN.