Uppsala/December 16, 2022 — OssDsign AB announced updated complaints data from a long-term follow-up of the company’s innovative product OssDsign Cranial PSI, which is used in the treatment of cranial bone defects. The data, based on 1,995 surgeries, shows that the frequency of infections leading to implant removal was 1.4% after an average follow-up time of 21 months. This positive outcome exceeds what has been observed in previous follow-ups, thus highlighting the exceptional performance of OssDsign Cranial PSI.
“The new data from the long-term post-market surveillance study of OssDsign Cranial PSI confirms previous results and strengthen OssDsign´s position in discussions with potential new partners as well as for further commercial growth. We are happy to continue delivering safe and effective products to patients in need of innovative solutions for skull defects,” commented Morten Henneveld, CEO of OssDsign.
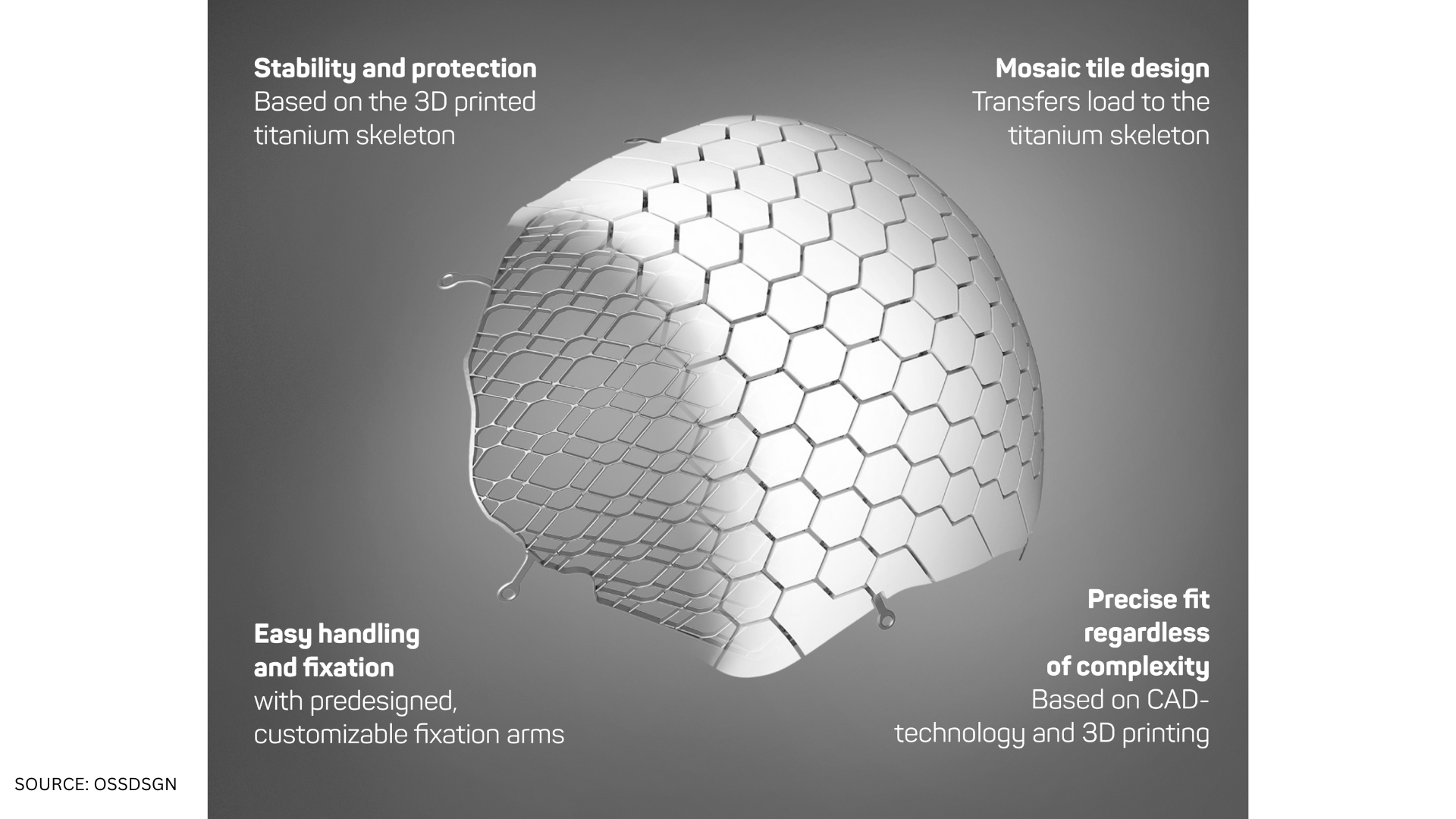
OssDsign Cranial PSI is a patient-specific cranial implant made from 3D printed medical-grade titanium covered by a regenerative calcium phosphate composition. While the titanium skeleton reinforces the implant and resists physical and mechanical stress, the unique calcium phosphate composition provides healing and regenerative properties, allowing regrowth of the patient’s own bone. Over time, the calcium phosphate composition degrades and is replaced with bone, leaving the patient with a well-integrated implant, potentially lasting a lifetime.
The results of the long-term follow-up show that complications in the form of infections leading to explantation of OssDsign Cranial PSI occurred in 1.4% (28) of 1,995 surgeries after an average follow-up time of 21 months (0-88 months). These positive results surpass those in the previous follow-up report, where the explantation rate was 1.6%. In earlier external studies, products available on the market have been shown to be associated with an infection complication rate of over 10% with a high rate of subsequent explantations.
The data were collected as part of post-market surveillance in Europe, the U.S., and selected Asian markets, in accordance with MEDDEV 2.7/1 rev. 4 and MDR 2017/745.


